Federal Intervention Urgently Needed to Address Pulse Oximeter Issues: Legal Action Suggested
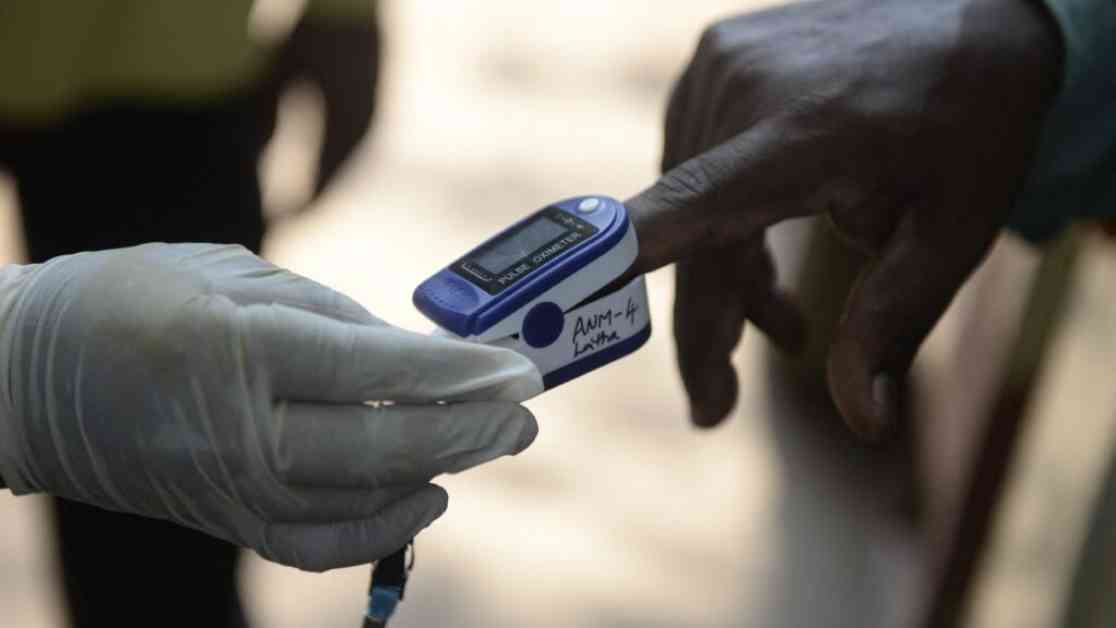
A groundbreaking study published in JAMA has shed light on the glaring disparities in the performance of pulse oximeters on individuals with darker skin tones, calling for immediate federal intervention to rectify this critical issue. Despite the FDA’s call for device manufacturers to diversify skin tone testing back in 2013, progress in improving oximeter accuracy for all skin tones has been alarmingly slow, leaving many patients at risk.
Insufficient Progress Despite FDA Recommendations
The study, led by researchers at Johns Hopkins University, meticulously analyzed FDA approval documents for 767 oximeters approved between 1978-2024, revealing a shocking lack of attention to skin tone variations. Only 4% of submissions made before the FDA’s diversity testing suggestion in 2013 referenced skin tone, a number that increased to a mere 25% post-guidance implementation. This inadequate response underscores the persistent challenges faced by darker-skinned patients in receiving accurate oxygen level readings.
Expert Voices Concern
Dr. Theodore J. Iwashyna, a critical care physician and study author, expressed deep frustration at the healthcare system’s failure to address this long-standing issue. “Our patients deserve better devices now,” emphasized Dr. Iwashyna, highlighting the urgency of the situation and the pressing need for improved oximeter performance across all skin tones.
Implications for Patient Care
The implications of inaccurate pulse oximeter readings are profound, particularly for marginalized communities. During the Covid-19 pandemic, oximeters were widely used to assess patients’ oxygen levels, leading to delays in treatment and higher mortality rates for individuals with darker skin tones. The urgency to address these disparities is further underscored by the disproportionate impact on Black patients, as evidenced by recent studies.
Call to Action
In response to mounting pressure, the FDA has taken initial steps to address the issue, including issuing warnings and convening advisory meetings. However, delays in releasing comprehensive guidance have sparked outrage among health equity leaders and frontline physicians who rely on oximeters for critical patient care decisions.
As conversations around legal action, federal intervention, and industry accountability gain momentum, the quest for equitable pulse oximeters remains a pressing priority. The road ahead may be challenging, but the imperative to ensure accurate and reliable devices for all patients is non-negotiable. The time for change is now.